Radiocarbon is widely used for dating geological and archaeological materials. Radiocarbon has also wide applications in studies of past and present environment.
Radiocarbon dating
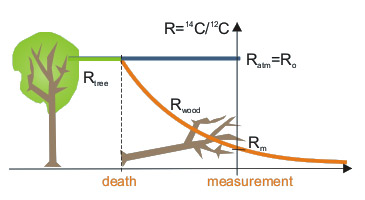
Due to assimilation from atmosphere, concentration of 14C in living terrestrial organisms remains constant (Ro). After death of organism, loss of 14C due to radioactive decay is no longer counterbalanced by assimilation, and amount of 14C starts to decrease.
Comparison of 14C/12C ratio in a sample of dead organic matter (Rm) with that in the atmosphere enables determination of radiocarbon age. This age means the time, which passed between death of organism and moment of measurement.
Past variations of 14C levels in the atmosphere
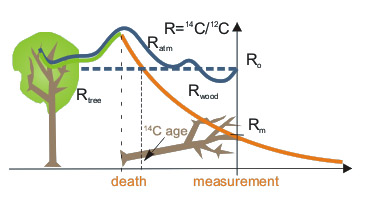
Radiocarbon age is determined with the assumption that the initial concentration of 14C (i.e. at the moment of death of an organism) was the same as in the standard of modern biosphere (with the correction for isotopic fractionation). This is equivalent to the assumption of constant 14C concentration in the atmosphere in the past. Determination of relationship between radiocarbon and calendar ages is the matter of calibration of the radiocarbon time scale.
Calibration of the radiocarbon time scale
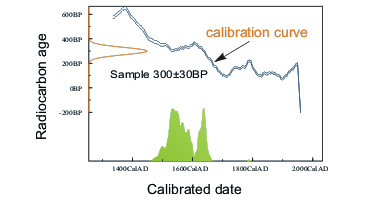
High-precision 14C dating of samples of known calendar age (mostly tree-rings, annual increments of marine and lacustrine sediments and corals) led to the construction of 14C calibration curve. This curve enables reading of calendar age if radiocarbon date is known. Such calendar age is expressed in “cal BP”, “cal BC” or “cal AD” units. Unfortunately, result of calibration is not always unequivocal (see picture to the left).
Useful computer program for calibration of radiocarbon dates may be downloaded e.g. from the website of the Oxford 14C Laboratory.
Reconstruction of calibration curve is equivalent to reconstruction of past variations of atmospheric 14C concentrations. This concentration is expressed in form of Δ14C, i.e. relative deviation from the concentration in the standard of modern biospere. Variations of atmospheric 14C are caused by geophysical (Earth’s magnetic field, water circulation in the oceans) and astropysical (activity of the Sun) factors. Among others, they provide information on fluctuations of solar activity in the past
All laboratories performing 14C measurements are registered and the scientists are joined in the community around “Radiocarbon” – International Journal of Cosmogenic Isotope Research.
See also:
isotopic fractionation, reservoir effect
