
At a first-order approximation, age of sample dated with radiocarbon may be determined by comparison of 14C/12C ratio in the analysed sample with that in the modern biosphere. The simplest assumption is that the 14C/12C ratio at the moment of organism’s death was the same as in the modern biosphere. This assumption, however, is not fulfilled, and in routine dating several corrections must be applied. The most frequent corrections concern: isotopic fractionation, past variations of atmospheric 14C and reservoir effect.
Isotopic fractionation
Isotopic fractionation terms dependence of rates of chemical reactions and physical processes on the mass of isotope. For example, all carbon atoms undergo photosynthesis, but for lighter carbon atoms this process is relatively faster. Therefore the ratio 14C/12C in plants is always smaller than in the atmosphere. This fact must be taken into account in 14C dating.
One suitable measure of fractionation is the ratio 13C/12C. For example, this ratio in natural carbonates is ca. 7‰ higher, while in most plants ca. 18‰ lower than in the atmosphere. Fractionation effects for 14C are twice as great as for 13C.
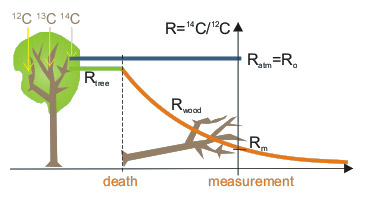
Magnitude of isotopic fractionation in fossil samples may be assessed by measurement of 13C/12C ratio. This ratio in organism also depends on isotopic fractionation and, as both isotopes involved (13C and 12C) are stable, it does not change after death of organism and may be measured in the laboratory. Measurement of 13C/12C in a sample enables correction of 14C/12C ratio, since in most processes fractionation for 14C is almost exactly twice that for 13C. As a rule, concentration of 14C is expressed after its normalisation to 13C/12C ratio in the standard of modern biosphere.
Isotopic fractionation occurs also during several stages of sample preparation for 14C measurements. Therefore the measured 14C/12C ratio must be normalised, using parallel 13C/12C measurement or measurement 13C/12C in sample prepared in identical manner.
Reservoir effect
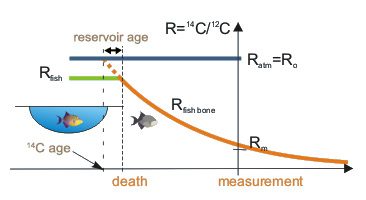
Correct radiocarbon age is most easily determined for remnants of organisms, which during their life derived carbon from the atmosphere. Organisms deriving carbon from aquatic environemnts usually have lower 14C/12C ratio than in the atmosphere. This is the so called “reservoir effect”. Radiocarbon age of such organisms’ fragments is usually too old and should be corrected by subtraction of “reservoir age”.
Reservoir age of surface water of seas and oceans is well known (300-800 years). In terrestrial water bodies (rivers, lakes) reservoir age may be very diverse (between 0 and 2000 years) and for each aquifer must be separately determined.
