
Carbon dioxide is produced from samples after chemical pretreatment. Organic samples are combusted, while carbonate samples are leached with acid. The released and purified CO2 is forwarded into graphitisation.

Combustion
The steps of combustion process are illustrated in the diagram below.
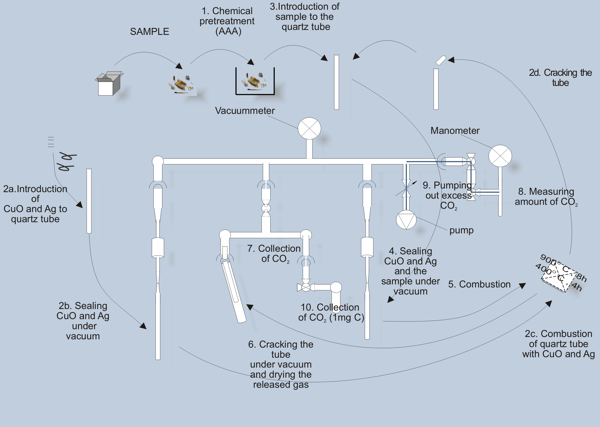
The sample is placed, and sealed under vacuum in quartz tube, together with an appropriate amount of copper oxide and silver (steps 3 and 4). The copper oxide serves as a source of oxygen necessary for combustion, while silver plays a role in CO2 purification. Before these steps, the walls of quartz tube and CuO and Ag are purified by backing under vacuum in high temperatues (steps 2a through 2d). For complete combustion, the tubes are kept in 900°C for 8 hours, and then in 400°C for 4 hours (step 5).
After combustion, the tube is cracked under vacuum, the released gas is dried (step 6) by freezing water in a cold trap (-80°C), and CO2 is collected in a trap cooled with liquid nitrogen (step 7). Amount of produced CO2 is determined by measurement of pressure in a known volume (step 8), and excess carbon dioxide is removed (step 9). Then the amount corresponding to 1 milligram of carbon is collected (step 10) for further processing.
The whole procedure, including purification of tubes and other components for the combustion, requires 4 days of work.
Acid leaching
Carbonate samples are leached with ortophosphoric acid, in 80°C. This is performed under vacuum, in a device similar to that used in combustion step 6. The released CO2 is purified and further processed in the same manner as in case of combustion (steps 7 – 10).
Other pages on sample preparation:
General – chemical pretreatment | CO2 production | Graphitisation
