
In this step, CO2 is converted into carbon (graphite). This carbon is then used as a cathode material in the AMS spectrometer.

CO2 is reduced in catalytic reaction with hydrogen. As a catalyst we use iron, placed in a quartz tube (step 11). The surface of iron is activated by subsequent oxidation and reduction (steps 12 and 13). Then, CO2 is introduced, and desired amount of hydrogen added (step 14).
The reduced carbon is deposited on hot surface of iron. To maintain the reaction, water vapour is removed continuously by freezing in a cold trap. The progress of the reaction is controlled with a manometer. When the reaction is completed (after 2 – 3 hours), the Fe-C mixture is pressed into the cathode holder (step 15).
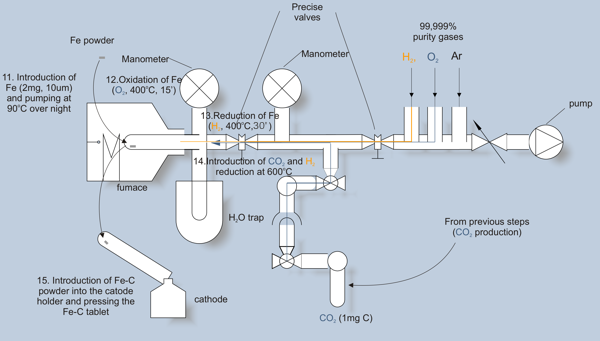
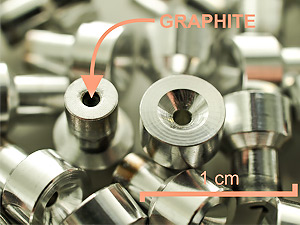
The cathodes are stored in atmosphere of argon, and ultimately forwarded into AMS measurement.
More details of our combustion procedures and consideration of contamination sources are published by Czernik and Goslar (Radiocarbon, vol. 43 (2001), in print)
Other pages on sample preparation:
General – chemical pretreatment | CO2 production | Graphitisation
