
Radiocarbon is a common name of 14C – the only radioactive isotope of carbon. Two main properties which differ 14C atoms from normal carbon atoms are: mass and radioactivity. Though in very low abundance, radiocarbon is widely distributed on the Earth. Radiocarbon is widely used for dating geological and archaeological materials. Radiocarbon has also wide applications in studies of past and present environment.
Masses of carbon isotopes
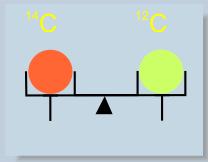
Isotopes are the groups of atoms of the same element and determined mass. Atoms of different isotopes have different masses. Most elements in nature have more than one isotope. Isotopes are denoted by a mass number left to the symbol of chemical element.
The element: carbon, has three isotopes – 12C, 13C, 14C. Atoms of all carbon isotopes have the same chemical properties, but one atom of 14C is 14/12 times heavier than that of 12C, and 14/13 times heavier than that of 13C.
Most carbon atoms are those of 12C. 13C is much less abundant (1 atom of 13C per about 113 atoms of 12C). Atoms of 14C are extremely rare – there is only one atom per ca. 1012 (1,000,000,000,000) atoms of carbon!
Radioactivity of 14C
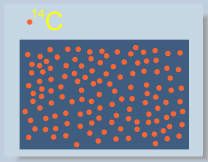
Atoms of 14C decay spontaneously. Decaying 14C atom produces one particle of beta radiation, and transforms itself into atom of nitrogen. This property is called radioactivity.
Because of decay, number of radioactive atoms in a certain portion of matter decreases with time. Number of radioactive atoms decreases by a factor of two after so called “half-life” time. Intensity of produced radiation dereases accordingly.
The half life time of 14C is 5730 years. Consequently, after 5730 years 1/2 of 14C atoms remain not decayed, after 11460 years this number decreases to 1/4, after 17190 – to 1/8, and so on.
Radiocarbon on the Earth
Though its half-life time is insignificant with respect to the geological time scale, radiocarbon is continuously present on the Earth. This is due to cosmic rays (mainly protons of extremely high energy, coming from the interstellar space), which interact with the atoms of atmosphere, and produce 14C from nitrogen. This is called cosmogenic production. On average, total radiocarbon production rate on the Earth is in equilibrium with the decay rate, and concentration of 14C remains constant.
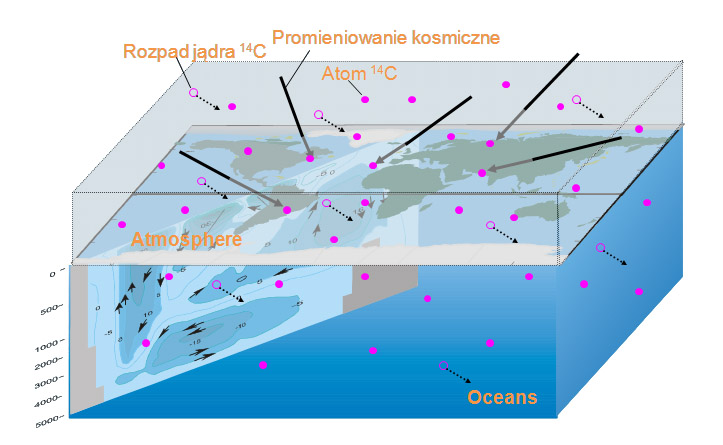
Once produced, 14C atom enters the carbon cycle. It circulates in the atmosphere mainly in form of carbon dioxide (14CO2). 14CO2 may be then assimilated into organic matter by plants. It may be dissolved and circulate with oceanic water. As the travel from atmosphere to deep oceans requires hundreds years, concentration of 14C in deep ocean is distinctly lower than in the atmosphere.
Isotopic ratio 14C/12C is usually quoted in relation to that in the worldwide accepted standard, called “standard of modern biosphere”. Consequently, concentration of 14C is commonly expressed in “percent of modern standard” (pMC).
